Philip Morris: World can eradicate cigarettes in 10 years
Philip Morris: World can eradicate cigarettes in 10 years
What does a world without cigarettes look like? This question is currently at the heart of debate by scientists, public health experts and sociologists from across the world in tandem with changing public attitudes towards smoking.
Part of the answer is coming from cigarette companies that have over years invested billions of dollars into research and development for alternative products reducing the risk to smokers.
Read also
“A future in which cigarettes are obsolete is within reach,” Philip Morris International CEO André Calantzopoulos recently told delegates at the 2020 Annual Concordia Summit held virtually.
“In fact, with the right regulatory encouragement and support from civil society, we believe cigarette sales can end within 10 to 15 years in many countries,”.
For decades now, cigarette companies have been working on developing alternative products to replace cigarettes.
As the scientific community acknowledges, although nicotine is addictive and not risk-free, it is the burning of tobacco that generates smoke containing harmful chemicals responsible for many smoking-related illnesses. The aim has therefore been to eliminate combustion.
One of these alternative products is IQOS, an electronically heated tobacco system developed by Philip Morris International s.
At the heart of the IQOS heating system is a sophisticated electronic unit that heats the tobacco just enough to release a nicotine-containing aerosol, contrary to cigarettes which burn tobacco reaching more than 600 °C, releasing high levels of harmful chemicals.
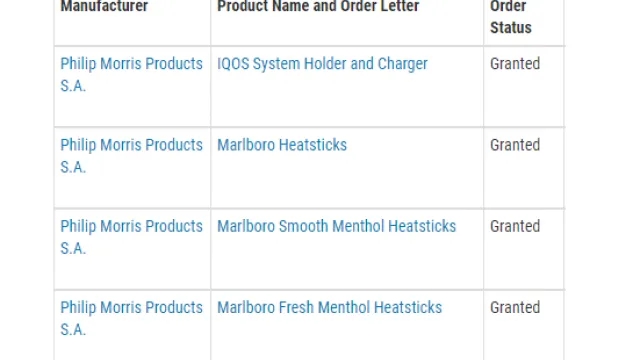
Thus, exposure to harmful chemicals is significantly reduced. In July this year Philip Morris International was authorised by the US Food and Drug Administration FDA to market the IQOS as a modified risk tobacco product (MRTP).
“This marks the second set of products ever to be authorised as MRTPs and the first tobacco product to receive “exposure modification’ orders, which permits the marketing of a product as containing a reduced level of or presenting a reduced exposure to a substance or as being free of a substance when the issuance of the order is expected to benefit the health of the population,’ said the FDA in its statement.
The decision by the FDA is a landmark one for the international firm that has been named second tobacco firm in the world in developing technology that reduces tobacco harm.
The FDA authorization further requires Phillip Morris International to continue conducting market research and share data with the regulators on how products like IQOS could help smokers transition to less harmful tobacco use while preventing recruitment of new smokers particularly among the youth.

“Through the modified risk tobacco product application process, the FDA aims to ensure that information directed at consumers about reduced risk or reduced exposure from using a tobacco product is supported by scientific evidence and understandable,’ said Mitch Zeller, J.D., director of the FDA’s Center for Tobacco Products speaking at a US conference on tobacco products’ regulation in October
Zeller further says data submitted by PMI shows that marketing products like the IQOS with the authorised information could help adult smokers transition away from combusted cigarettes and reduce their exposure to harmful chemicals, but only if they completely switch.
“Studies showed switching completely from combustible cigarettes to the IQOS Tobacco Heating System significantly reduces the body’s exposure to 15 specific harmful potentially harmful chemicals,’ said the FDA in its statement.
“The toxicological assessment also found that, compared with cigarette smoke, IQOS aerosols contain considerably lower levels of potential carcinogens and toxic chemicals that can harm the respiratory or reproductive systems,” read the statement in part.
PMI hopes the IQOS will be the first in a diverse portfolio of potentially less harmful products that can help switch an even greater number of adult smokers and make a decisive impact towards improving public health in many countries.
As regards IQOS presence in the continent last year the South African unit of PMI opened the first flagship store in Sandton, Johannesburg specifically catering to selling alternative heated tobacco products.
This followed the launch of a pilot store in Cape Town in 2017 that operated for a year to test market demand. PMI says the store will be re-opened in the future on a permanent basis and that plans are underway to roll out other stores across the continent.



