Kenya greenlights twice-yearly HIV prevention shot Lenacapavir
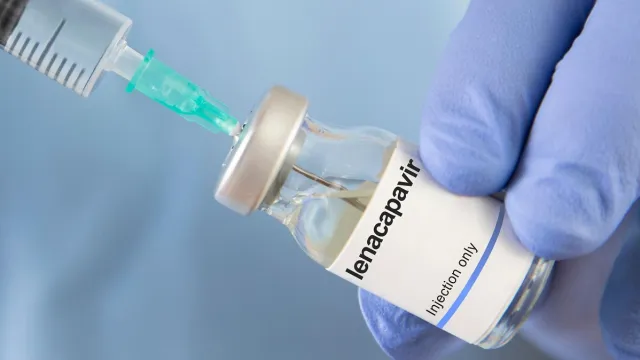
According to WHO, with just two doses per year, lenacapavir has been established to offer a transformative step forward in protecting people at risk of HIV – particularly those who face challenges with daily adherence, stigma, or access to health care.
Authorities in Kenya have approved the use of lenacapavir, a HIV prevention drug that is administered twice per year for use in managing the disease. According to an update by the Ministry of Health at the weekend, the approval followed relevant scientific assessment of the drugs quality, safety, and efficacy.
"Its long-acting formulation allows it to be administered only twice a year, offering an important alternative to daily oral HIV prevention medicines. This is particularly beneficial for individuals who face challenges with taking pills every day," noted Health Cabinet Secretary Aden Duale in a statement.
Analysis shows that Kenya is a pioneer across Africa in allowing the use of lenacapavir, and the decision is in harmony with the latest guidance on public health including by the UN's World Health Organization, explained Duale.
Additionally, the CS said the approval is in harmony with Kenya's expanding health regulatory capacity and leadership that is increasingly facilitating access to innovative health technologies in a timely manner.
Duale said the ministry will ensure the medicine is introduced in a timely, equitable and responsible manner for populations at substantial risk of HIV infection.
In July last year, the WHO said the first twice-yearly injectable drug, offers pateints a highly effective, long-acting alternative to daily oral pills and other shorter-acting options.
According to WHO, with just two doses per year, lenacapavir has been established to offer a transformative step forward in protecting people at risk of HIV – particularly those who face challenges with daily adherence, stigma, or access to health care.
“While an HIV vaccine remains elusive, lenacapavir is the next best thing: a long-acting antiretroviral shown in trials to prevent almost all HIV infections among those at risk," said Dr Tedros Adhanom Ghebreyesus, WHO Director-General.
“The launch of WHO’s new guidelines, alongside the FDA’s recent approval, marks a critical step forward in expanding access to this powerful tool. WHO is committed to working with countries and partners to ensure this innovation reaches communities as quickly and safely as possible.”
The latest HIV and AIDs data in Kenya shows that the country experienced a 19 percent increase in new HIV infections, with cases hitting 19,991 in 2024 from a lower 16,752 recorded in 2023.