New HIV prevention shot could be a game changer — here’s why
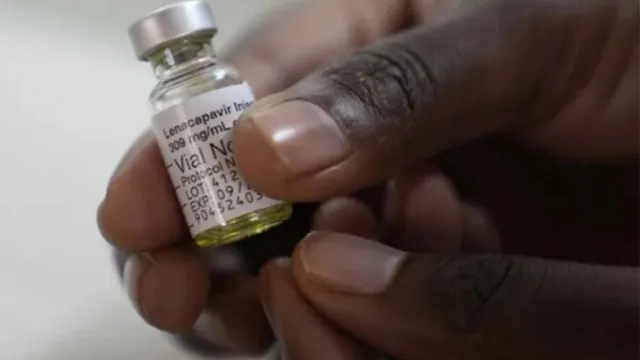
Gilead Sciences' new drug, lenacapavir, a twice-yearly injection, helps in preventing HIV infection in both adults and adolescents.
The U.S. has approved the world’s longest-lasting type of vaccine to prevent HIV transmission, marking the first step in an anticipated global rollout that could protect millions and bring reprieve to thousands of Kenyans
The US Food and Drug Administration approved Gilead Sciences' new drug, lenacapavir, a twice-yearly injection, for preventing HIV infection in adults and adolescents. Lenacapavir belongs to a new class of antiretrovirals known as capsid inhibitors. In large-scale clinical trials conducted last year, the drug demonstrated nearly 100 percent effectiveness in preventing HIV transmission.
Notably, the two shots in a year offer an alternative to daily oral pills, significantly reducing the burden of taking medication and lowers risks of defaults. It’s given as two injections under the skin of the abdomen, leaving a small “depot” of medication to slowly absorb into the body.
This new option could provide a much-needed lifeline for those who have failed other treatments or developed resistance to the already available options.
However, it is important to note that it only prevents HIV transmission and does not block other sexually transmitted diseases. Gilead also warned that people must test negative for HIV before getting their twice-a-year dose.
While a vaccine to prevent HIV is still needed, some experts say the shot made by Gilead Sciences could be the next best thing. It nearly eliminated new infections in two groundbreaking studies of people at high risk, better than daily preventive pills they can forget to take.
“This really has the possibility of ending HIV transmission,” said Greg Millett, public policy director at amfAR, The Foundation for AIDS Research.
WHO estimates that nearly 40 million people globally are living with HIV. Kenya, which has been among the countries that have suffered a shortage of HIV life-saving drugs precipitated by a decision by the US to pause foreign aid in January and ranks seventh globally in HIV burden with an estimated 1.4 million people living with the virus, is expected to be among the key beneficiaries if access to the drug is expanded to low- and middle-income countries, alongside other 18 nations that include South Africa and Nigeria.
This is because drugmaker Gilead Sciences said it will allow cheap, generic versions to be sold in 120 poor countries with high HIV rates with priority zones being mostly in Africa, Southeast Asia and the Caribbean, late last year.
They signed non-exclusive, royalty-free voluntary licensing agreements with six pharmaceutical manufacturers to make and sell generic lenacapavir, subject to required regulatory approvals, in 120 high-incidence, resource-limited countries. These are primarily low- and lower-middle income countries.
The agreements were signed in advance of any global regulatory submissions to enable these countries to quickly introduce generic versions of lenacapavir for HIV prevention, if approved. The approved drug, Lenacapavir, will be marketed under the brand name Yeztugo.